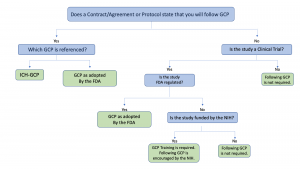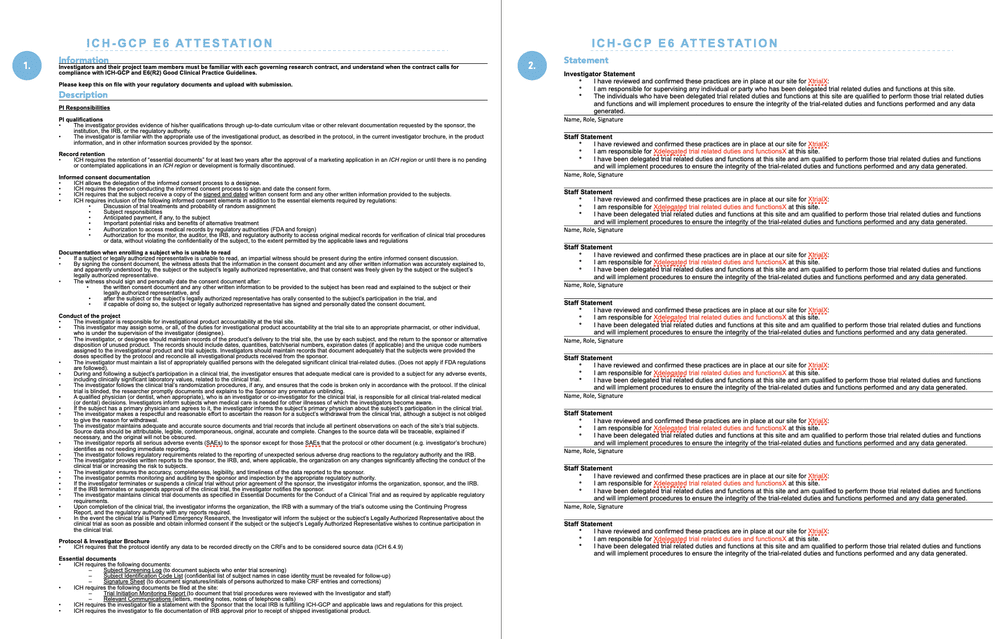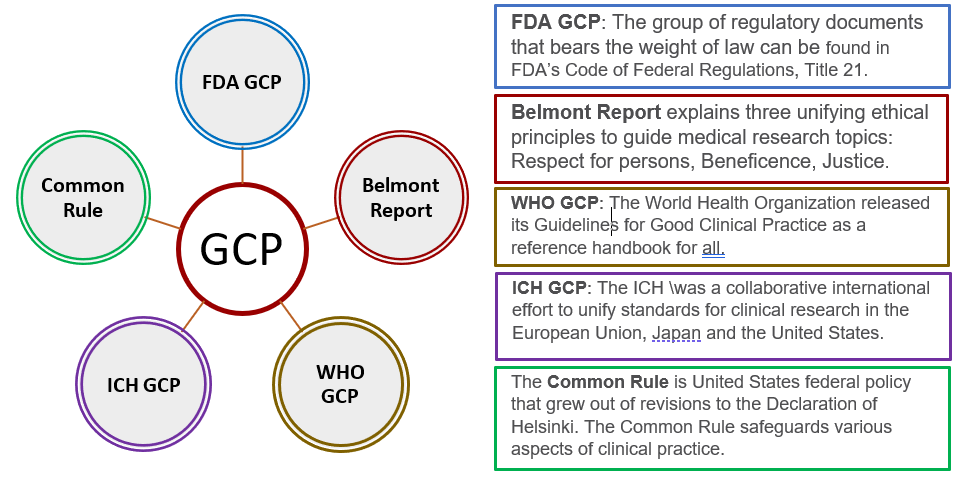
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering

The Good Clinical Practice guideline and its interpretation – perceptions of clinical trial teams in sub‐Saharan Africa - Vischer - 2016 - Tropical Medicine & International Health - Wiley Online Library

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram