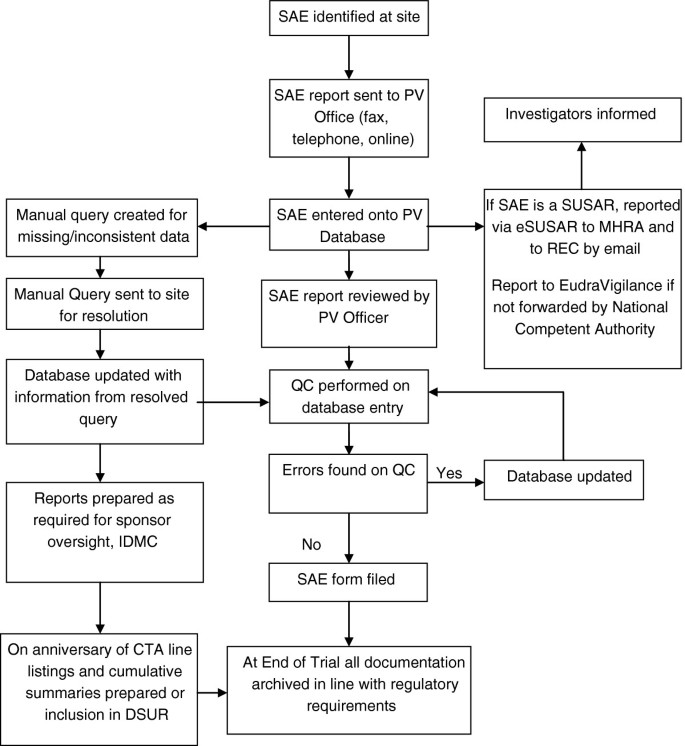
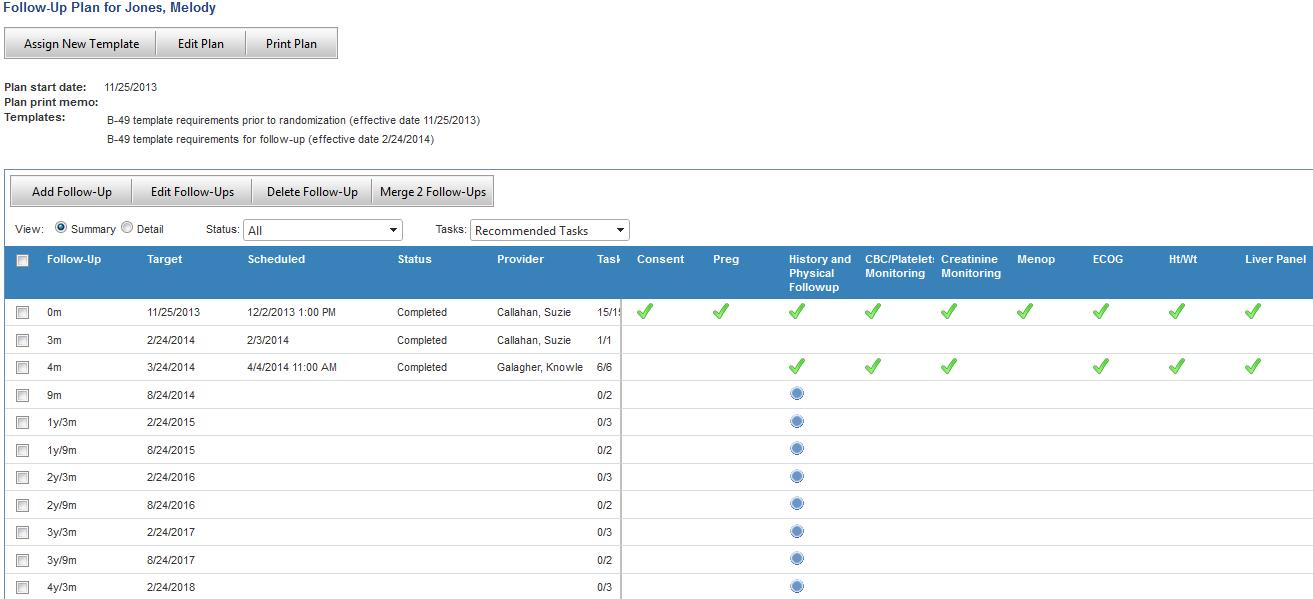
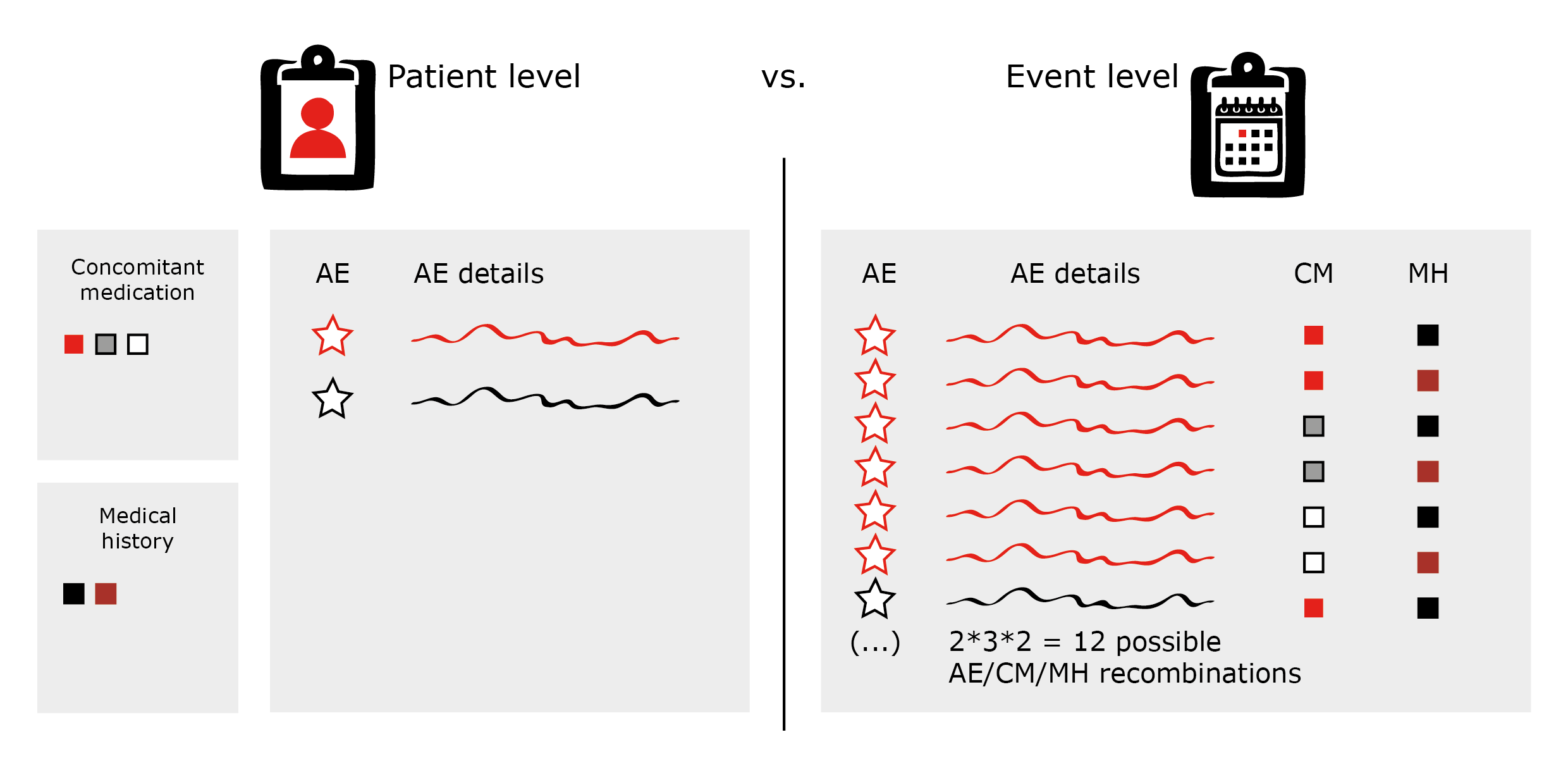
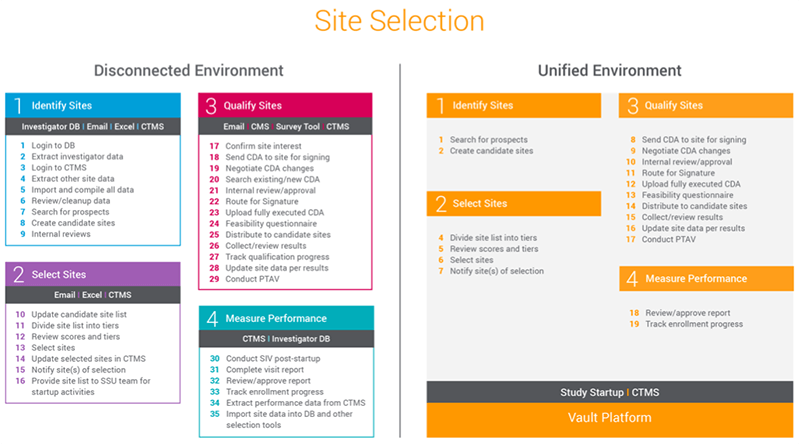
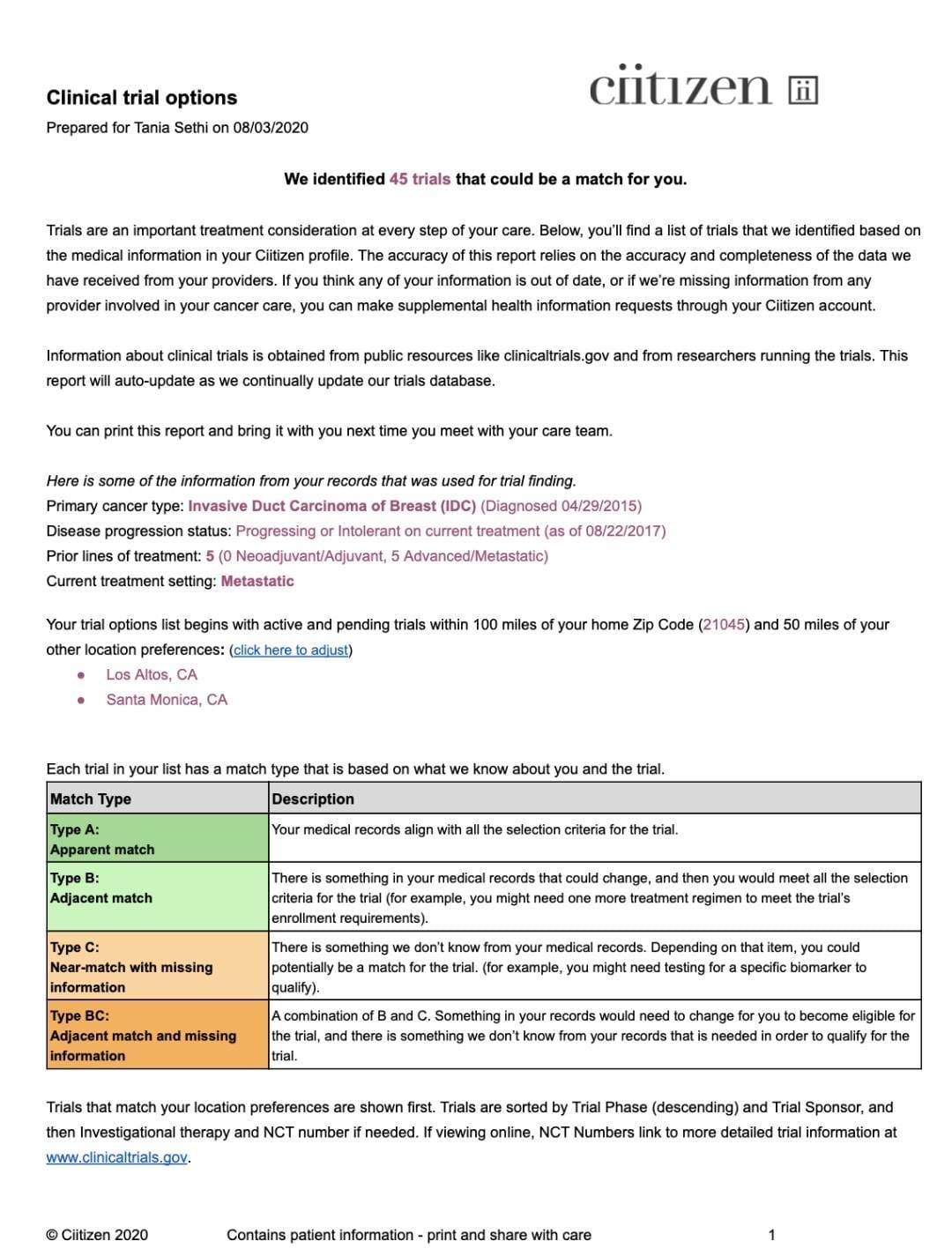
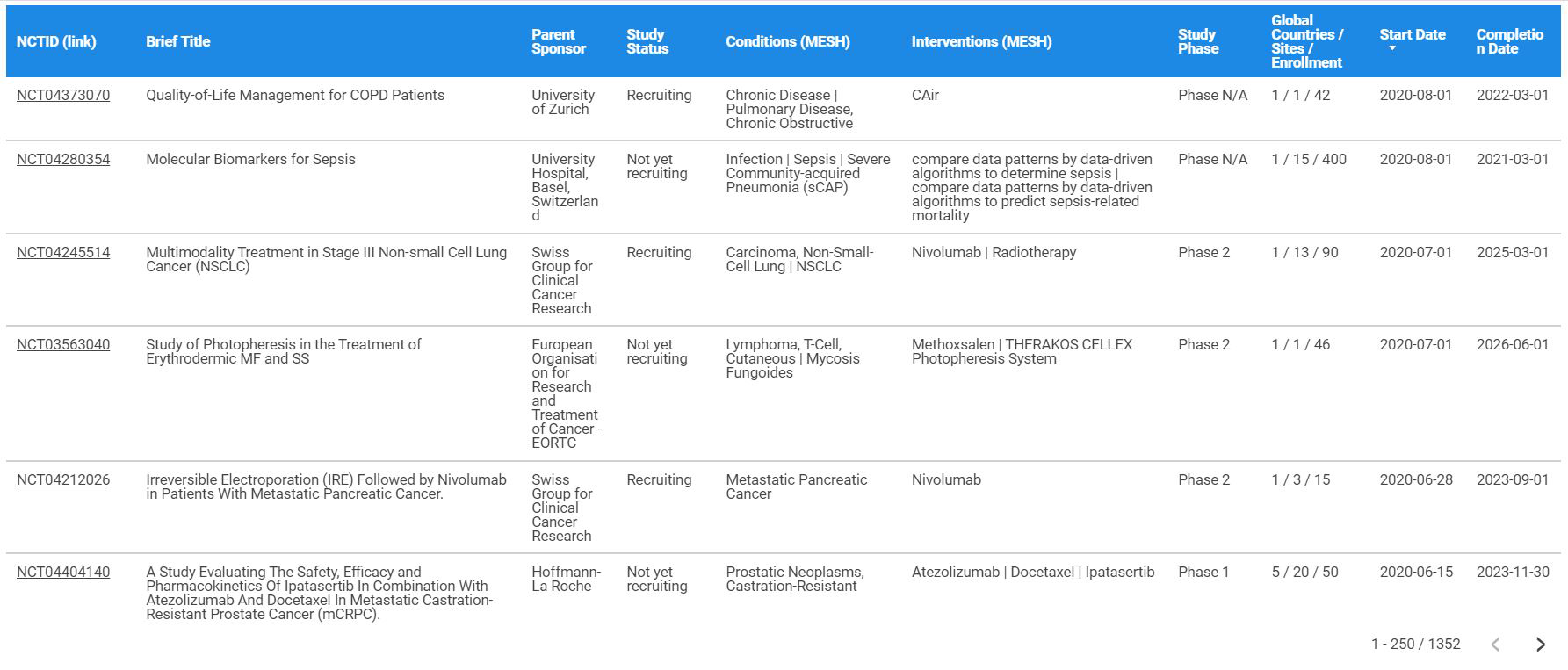
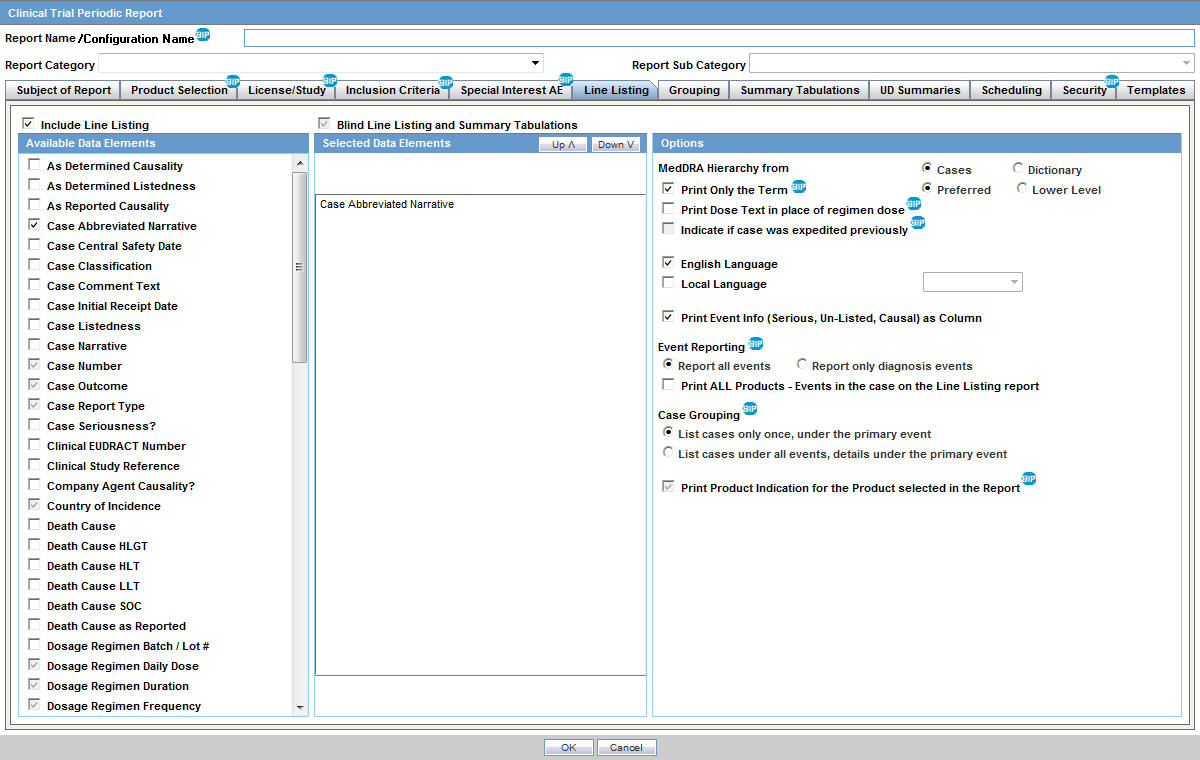
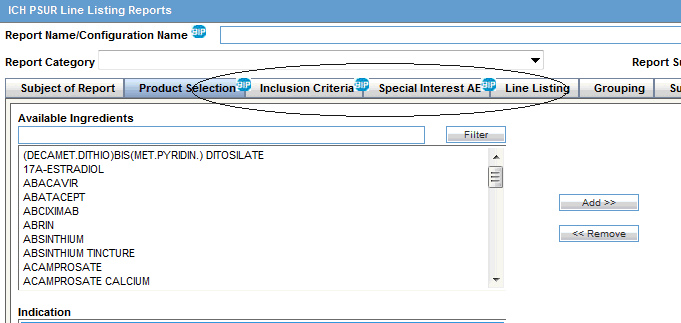
Jack of all Listings, A New Approach for Review of Clinical Data Hardik Panchal, Celgene Corporation, NJ
Jack of all Listings, A New Approach for Review of Clinical Data Hardik Panchal, Celgene Corporation, NJ

Differences in reporting serious adverse events in industry sponsored clinical trial registries and journal articles on antidepressant and antipsychotic drugs: a cross-sectional study | BMJ Open

A COVID-19 Vaccine Needs the Public's Trust—And It's Risky to Cut Corners on Clinical Trials, as Russia Is | The Pursuit | University of Michigan School of Public Health | Coronavirus